SPECIFIC HAZARDS INCLUDING ATMOSPHERIC HAZARDS
A combination of hazards associated with the confined space regulations are addressed, which include the nature of the confined space; the possible presence of substances or conditions, which together make for an increased risk to the health and safety of people.
Bodycote HIP Ltd was fined £533,000 and ordered to pay costs of £200,000 on 24th July 2009 for breaching section 2(1) of the Health and Safety at Work etc Act 1974 following the deaths at its manufacturing plant in College Road, Hereford.
The court heard that on the 14th June, 2004, the company’s Works Manager and Maintenance Engineer were found collapsed on the stairs leading to a concrete- lined pit into which argon gas had leaked from a large pressure vessel. The pit’s oxygen alarm system was switched off and the ventilation system was not running.
HSE Inspector Luke Messenger said: “Both these tragic deaths were not only regrettable but also entirely preventable. The risks from confined spaces and asphyxiation due to the presence of argon were well known to the company, which had experience of a similar double fatality at a Bodycote Group site in California, just three years earlier.
“Despite this warning the company failed to undertake a proper risk assessment for entry into the confined space. Although they had implemented a safe system of work and permit to work procedure, they had not properly trained employees in their use, or ensured that these systems and procedures were being followed through their auditing procedure. On the day of the incident, the ventilation system, which could have removed the leaking argon before it became a problem, and the oxygen alarm system, which would have warned of the oxygen-depleted atmosphere, were not switched on. Had these systems been working these two deaths may not have occurred.
Flammable substances and oxygen enriched atmospheres – Flammable gases occur naturally through organic decomposition of dirt and sludge. Alternatively, a flammable gas can be introduced by an external source. Additionally the risk of fire or an explosion can occur from the presence of flammable substances or an oxygen enriched atmosphere, i.e. leakage from an oxygen cylinder forming part of the welding equipment or leakages from adjourning plants or processes. Flammable gases are also produced by degradation of
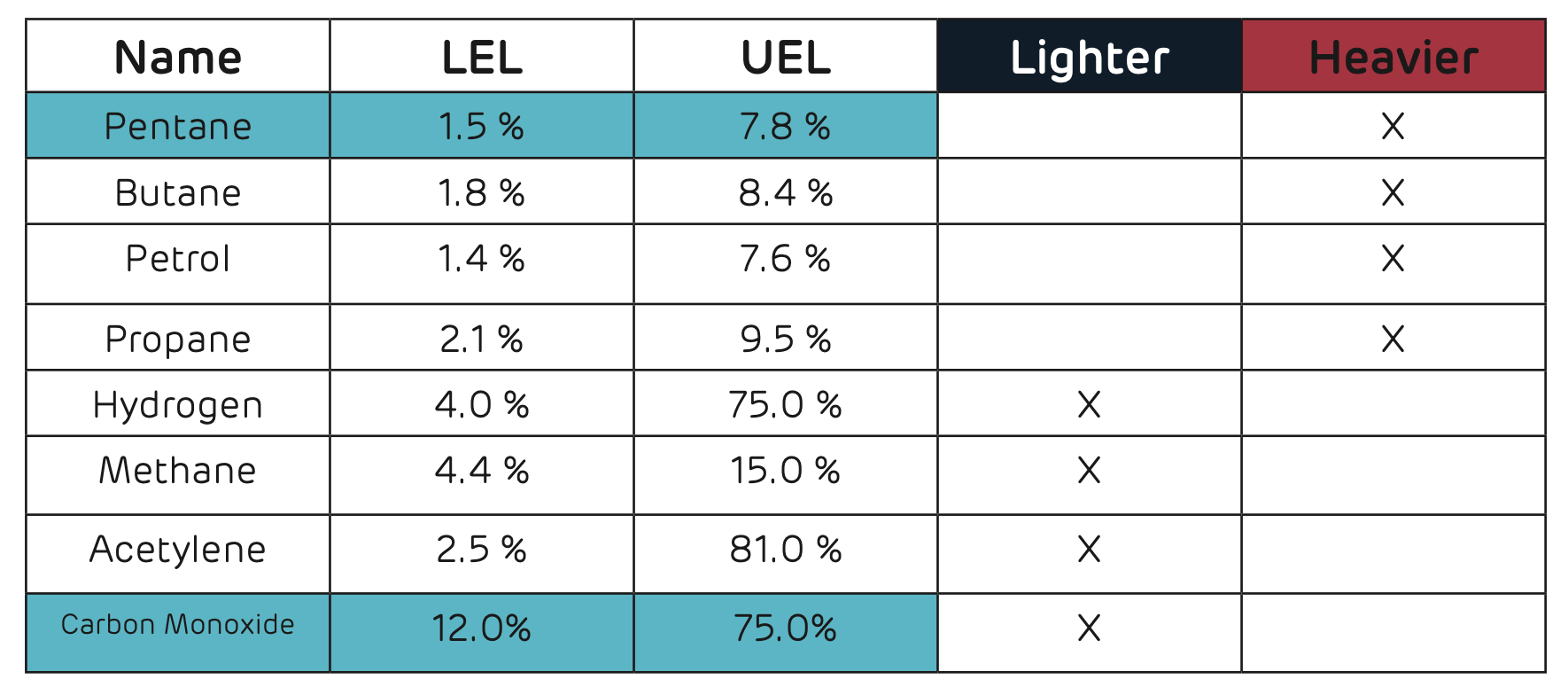
EXAMPLES OF FLAMMABLE GASES INCLUDE;
- Methane which is produced via sewage, rotting vegetation and natural gas
- Ammonia – refrigeration plants
- Petroleum – Spillages, residues, trade waste and road traffic incidents
- Acetylene – Welding operations
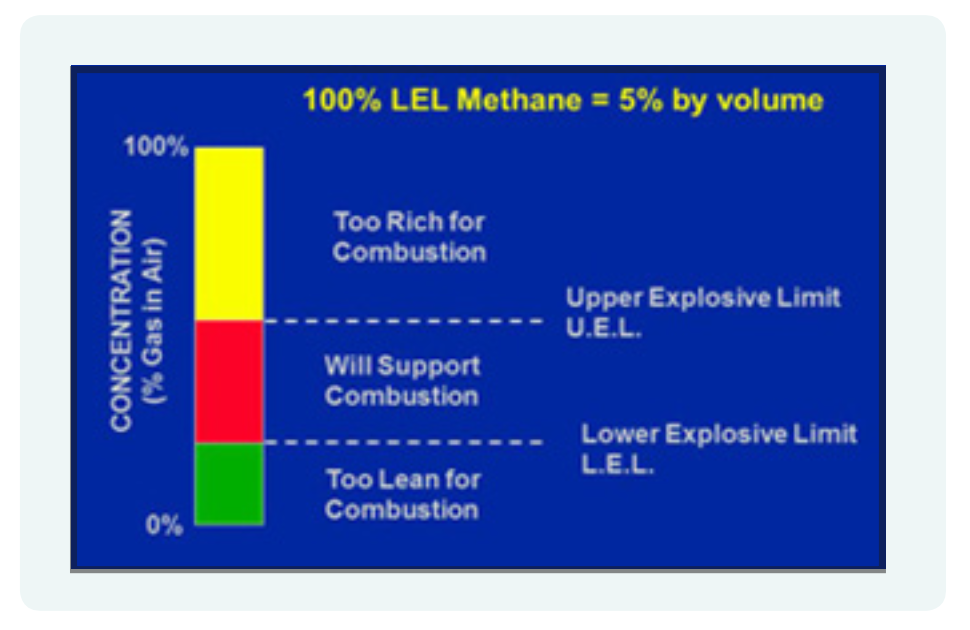
For a flammable atmosphere to become explosive, a mixture must be formed containing the appropriate amounts of fuel and oxygen. This term is known as its flammable range or limits of flammability. These terms are further explained below;
Lower Explosive Limit (LEL) is defined “in relation to a flammable contaminant, the concentration of the contaminant in air below which the propagation of a flame does not occur on contact with an ignition source.”
Upper Explosive Limit (UEL) is defined “in relation to a flammable contaminant, the concentration in air above which the propagation of a flame does not occur on contact with an ignition source.”
Ideal Mixture or Stoichiometric Mixture is defined as the ideal mixture in which all the fuel and oxygen are used and produce the most aggressive and forceful reaction.